This is Part 2 in a Series on the Climate Impact of Methane. See Part 1, “Is Methane From Cows the Same for the Climate as Methane from Fossil Fuels?” here.
WTF is a Hydroxyl Radical?
One complicating factor shaping Methane’s warming influence is it’s changing lifetime. The longer its average lifetime in the atmosphere, the more time it will have to warm the atmosphere. The shorter its average lifetime, the less time it spends warming the atmosphere.
Its lifetime can change as a result of shifts in the most typical methane sink, called the Hydroxyl Radical. So here’s a story about the potential change in the amount of Hydroxyl Radical (OH) in the atmosphere, and its influence on Methane:
Today, after being emitted, most Methane (CH4) molecules live about 12 years in the atmosphere before being broken down by a short-lived molecule called Hydroxyl Radical (OH) - a molecule which typically only lives for about… 1 second!
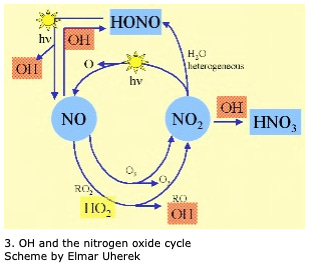
OH is typically formed by a two-step process in the atmosphere: First, Ozone (O3) reacts with UV light, splitting into O2 + O. Then the rogue Oxygen atom reacts with water vapour (H2O) to create two Hydroxy Radical (OH) molecules. I like to think of this as wandering floating Ozone molecule suddenly getting slammed with bit of sunlight, which causes it to split, as in this diagram:
However, OH can ALSO be formed by "the photolysis of nitrous acid (HONO), hydrogen peroxide (H2O2), or peroxy-methane (CH3OOH); or the reaction of nitrogen monoxide (NO) with the hydroperoxy radical (HO2)" which is a very complicated way of saying that OH is sensitive to Nitrogen Oxide... which we'll come back to shortly... but for now park that away in a corner of your brain.
OH is known as the "detergent of the atmosphere" as it helps to oxidize (aka break down) trace gases like Methane (CH4) and Carbon Monoxide (CO)... this process also destroys the OH in turn. Most OH destruction (about 75%) is from its reaction with CO (generating CO2 and H in turn).
Most of the remainder of OH destruction comes from its reaction with CH4. (In that process it generates water and a methyl radical, CH3). This generates a series of reactions, resulting eventually in HCHO (natural formaldehyde!).
Formaldehyde in turn breaks down into formic acid (HCOOH) and carbon monoxide (CO). The CO then reacts with more OH to form CO2 and H (as noted above!). The formic acid can also eventually breakdown into CO and water.
Who cares?
Because of all this, methane concentrations in the atmosphere are not only driven by anthropogenic emissions (and fluctuating natural emissions), but also they are driven by our indirect influence on the OH sink! The larger the OH sink, the more CH4 gets broken down. The more CH4 gets broken down, the shorter its average lifetime. The shorter its lifetime, the smaller the warming impact of methane emissions.
One recent study shows how human emissions of Nitrogen Oxides (NOx) and Carbon Monoxide therefore play a large indirect role in Methane concentrations by influencing the size of the OH sink. As the authors of this study write, "Increases in NOx tend to increase OH" while "increases in CO tend to decrease OH".
During the pandemic lockdowns of 2020, emissions of NOx and CO both reduced significantly. The Net effect, however, was a reduction in the OH sink (and unfortunately, a greater Methane lifetime, which likely helped explain the sizeable annual increases in the global concentration of methane that we’ve seen in recent years.
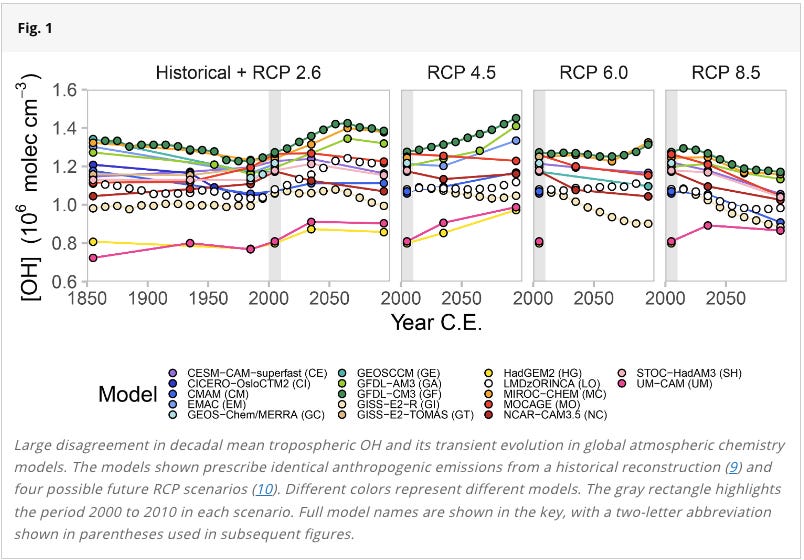
Unfortunately, it’s very difficult to predict the future size of the global OH sink. There are so many darned variables and feedbacks, including how future warming might impact water vapour, and how human pollution trends evolve.
This study shows how models of the OH sink disagree by + or - 30% into the coming decades. This means that Methane’s average lifetime in the future may be longer, but it also may be shorter.
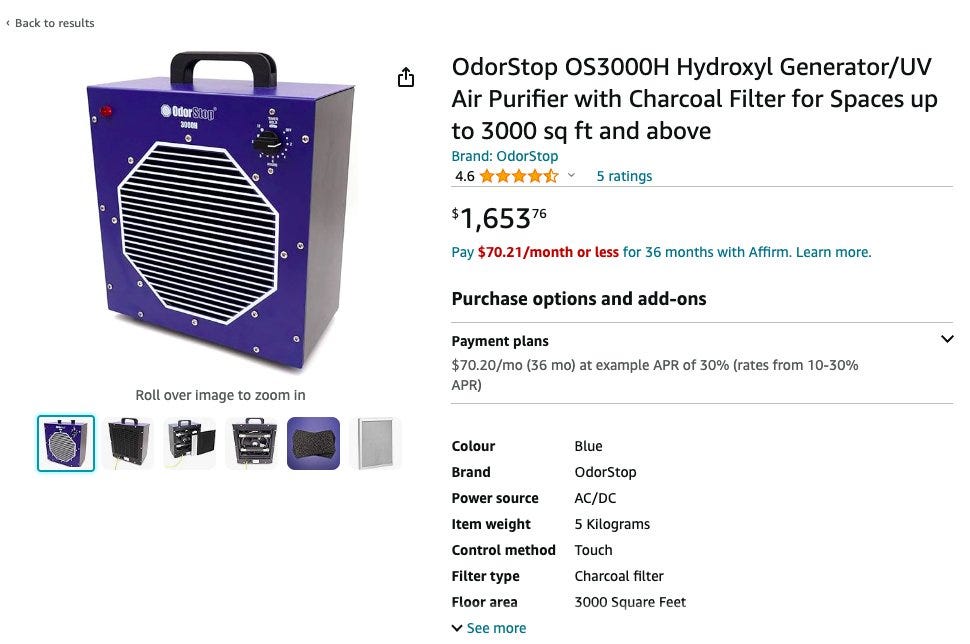
I know what you're thinking now... If human pollution can influence the OH sink, can we 'geoengineer' this in some way to produce more OH, and thereby reduce CH4 on the tail end on purpose? The short answer is "technically yes". In fact, you can go to Amazon.com right now and buy an hydroxyl generating device yourself, today for a small fee of $1654!
Another scientific paper has explored a number of prototype designs to produce much more meaningful quantities of OH, including one prototype for a solar-powered seawater powerplant which inadvertently produces OH, and... solar powered OH-generating kites! Again, these scientific efforts seek to reduce CH4 concentrations - not by reducing CH4 emissions - but by increasing the size of the OH methane sink.
Who knows, maybe some genius out there will discover an innocuous way to generate large quantities of OH, thereby enabling a reduction in global CH4 levels. Until then though, we may have to continue along the path of CH emissions reductions (see Part 1 of this series)!